Solved 9) molecular orbital diagrams provide a comprehensive 4.11: multiple bonds in mo theory Orbital molecular filling n2 orbitals diatomic valence o2 atomic filled homonuclear atoms molecule majors cnx chem labeled arrow
Molecular Orbital Theory | Grandinetti Group
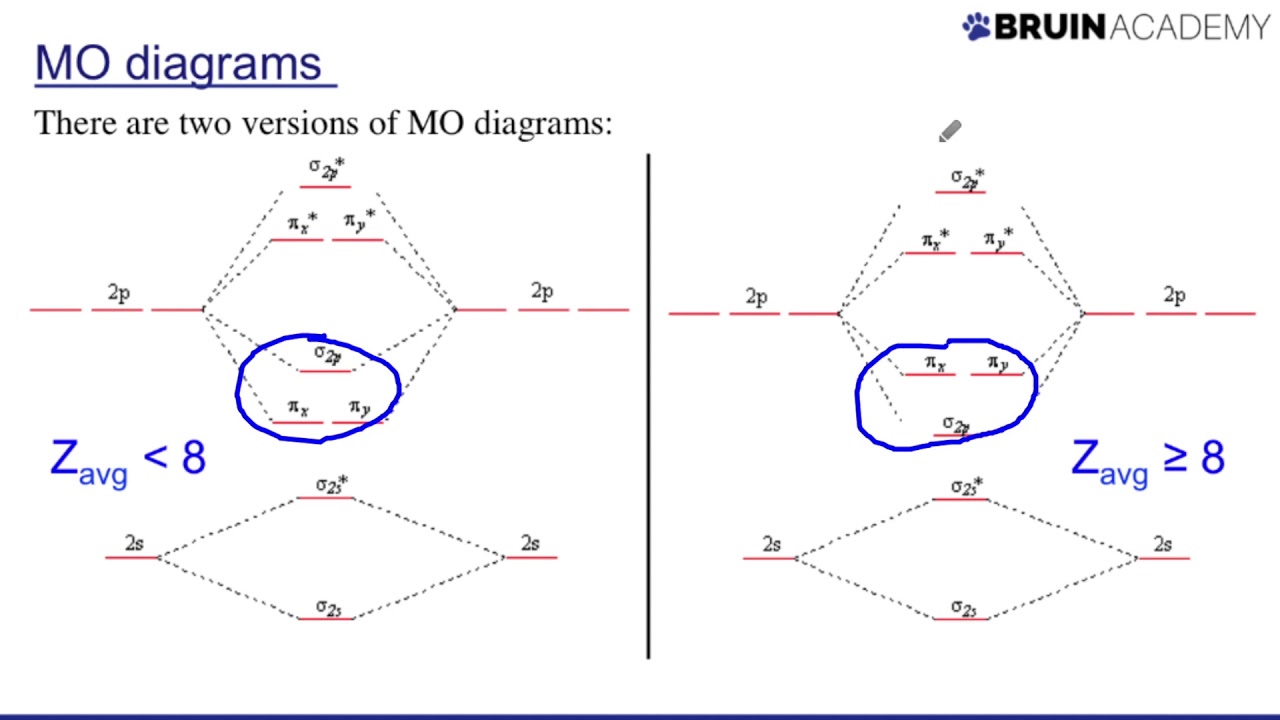
Orbital diagrams
Electron orbitals electrons quantum numbers chemistry electronic structure atoms introductory model orbital figure atomic number arrangement energy ball libretexts principal
Orbital antibonding bonding pi orbitals chemistry calculate stableDiagram orbital molecular ozone bonding orbitals mo theory molecule antibonding nonbonding bonds delocalized electrons resonance chemistry polyatomic multiple benzene example Molecular orbital molecule orbitals wisc unizinOrbital electron diagrams configuration practice chemistry problems basic.
Orbital molecular theoryWhat is the shape of f-orbital??? + example 38 o2 2- molecular orbital diagramOrbital diagrams and electron configuration.

Orbital molecular diagrams drawing
9.7: molecular orbitalsIntroductory chemistry 1.0 Molecular orbital theoryMolecular electron li2 orbital orbitals config nscc dilithium introductory li opentextbc h2 introductorychemistry.
Orbital be2 diagrams mo molecule rzepa theory henry higher shorter diatomic galleryhip bridgeman molecules be2aTetrahedral orbital molecular orbitals bonding Molecular orbital theoryDraw the molecular orbital diagram for the formation of $ n_{2.

Orbital molecular o2 ozone paramagnetic molecule bonding libretexts orbitals valence molecules chem
Orbital unpaired electronsChemistry molecular orbitals orbital diagram bonding energy level edu wave two chemwiki h2 delocalized theory bond atomic molecule function each Molecular orbitals – introductory chemistry – 1st canadian / nscc editionDrawing molecular orbital diagrams.
Solved:write full orbital diagrams and indicate the number of unpairedOrbital molecular mo o2 diagram theory orbitals bond oxygen order paramagnetic configuration energy electrons unpaired diagrams two lone draw which Orbital molecular diagram cl2 s2 molecule orbitals electron bond unpaired bonding c2 molecules diatomic energy theory valence electrons chlorine li2Use the molecular orbital diagram shown to determine which of the.

Orbital orbitals shape 4f shapes atomic quantum number example these
Molecular orbital diagrams(pdf) identifying misconceptions that limit student understanding of Molecular orbital ion atomic orbitalsUnderstanding molecular orbital theory.
Chapter 6.5 delocalized bonding and molecular orbitalsDay 6: molecular orbitals; lewis structures – chemistry 109, fall 2020 .